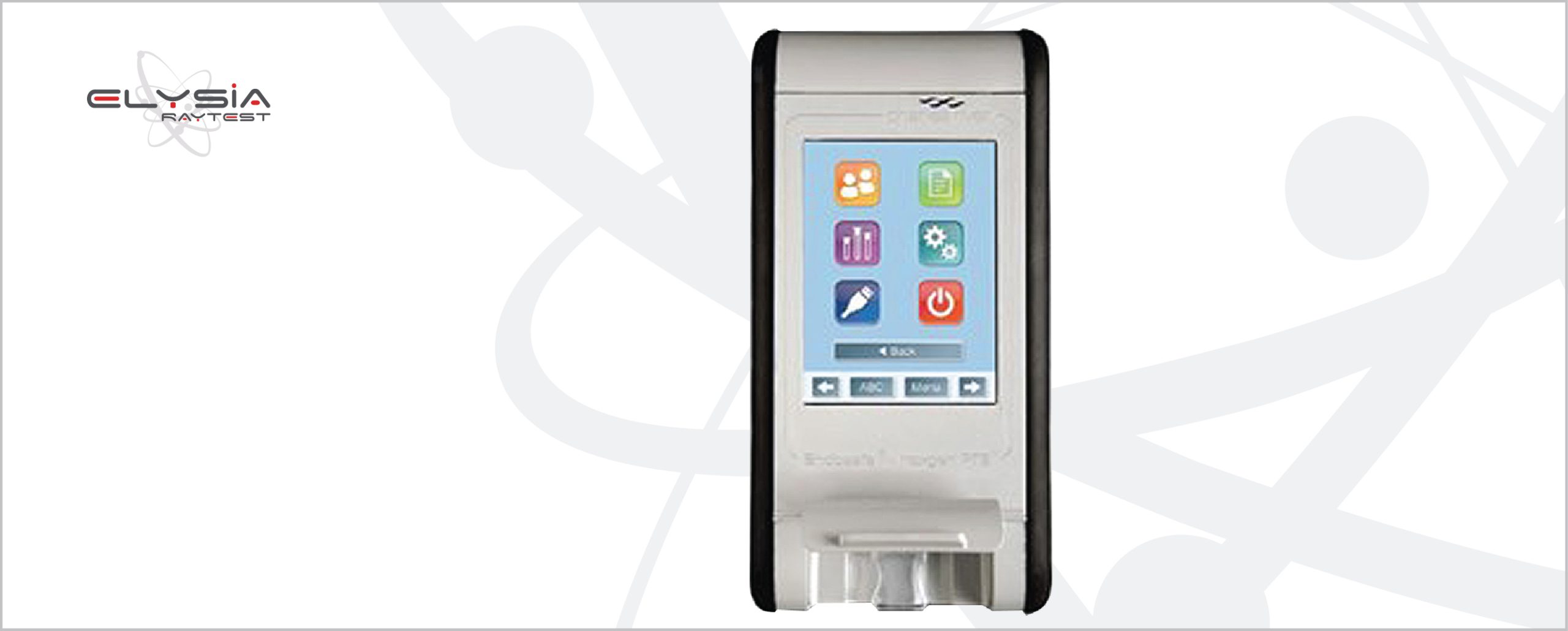
Endosafe Nexgen-PTS™
Rapid Endotoxin Detection System
The Endosafe Nexgen-PTS™ is an FDA-licensed endotoxin detection system designed for the routine quantitative determination of endotoxins. It provides a rapid, point-of-use testing solution capable of delivering accurate quantitative results in less than 15 minutes, enabling fast and reliable quality-control decisions in pharmaceutical and radiopharmaceutical environments.
The system utilizes licensed Limulus Amebocyte Lysate (LAL) reagents contained within a single-use disposable cartridge, processed by a compact handheld reader. This configuration creates a fully contained, real-time endotoxin assay suitable for laboratory testing, in-process control, and point-of-sample-collection applications.
Technology and Measurement Principle
Endosafe operates using kinetic chromogenic LAL methodology, in which the intensity of color development is directly proportional to the endotoxin concentration in the tested sample.
Each disposable cartridge contains:
• Precisely measured LAL reagent
• A chromogenic substrate
• A control standard endotoxin (CSE)
Cartridges are manufactured under FDA-licensed cleanroom conditions compliant with cGMP, ensuring high accuracy, consistency, and stability of results.
Test Procedure
The Endosafe testing workflow is simple and standardized:
1. 25 µL of sample is pipetted into each of the four cartridge reservoirs.
2. The reader automatically mixes the sample with LAL reagent in sample channels and with LAL reagent plus positive product control in spike channels.
3. Following incubation and chromogenic reaction, the system measures optical density.
4. Quantitative endotoxin values are calculated using an internally stored standard curve.
Key Performance Characteristics
Speed and Ease of Use
• Quantitative endotoxin results available within 15 minutes
• Simple operation requiring minimal user training
• Suitable for quality-control laboratories, in-process testing, and point-of-use environments
Portability and Connectivity
• Handheld, battery-powered reader with approximately 4–6 hours of operation
• Wireless data transfer and download to a central computer for tracking and trending
• Compatible with turnkey QC solutions and data management systems
Sensitivity and Compliance
• Four selectable sensitivity ranges: 0.005, 0.01, 0.05, and 0.10 EU/mL
• USP/EP BET-compliant, FDA-licensed cartridges
• Supports 21 CFR Part 11 compliance for regulated environments
Hardware and Technical Specifications
• Temperature control: 37 °C ± 1 °C
• Warm-up time: approximately 5–10 minutes from room temperature
• Display: large LCD touch screen
• User management: three password-protected access levels (administrator, manager, user)
• Data storage: 8 GB capacity, capable of storing thousands of reports
• Interfaces: USB, secure Wi-Fi, and network printer connection (RJ-45)
• Printer compatibility: thermal printer support
• Power supply:
– External: 100–240 V, 50–60 Hz
– Internal: 12 V DC, 5 A
• Battery life: approximately 4–6 hours after full charge
Applications
The Endosafe Nexgen-PTS™ is designed for:
• Routine endotoxin determination in pharmaceutical and radiopharmaceutical production
• In-process control, raw-material testing, and product release testing requiring rapid results
• Quality-control laboratories and point-of-use environments where immediate microbiological assessment is essential
Its speed, portability, and regulatory compliance make it particularly well suited for radiopharmaceutical production facilities and other time-critical manufacturing settings.
Advantages
• Rapid quantitative endotoxin detection in under 15 minutes
• Fully contained disposable cartridge technology
• High accuracy, reproducibility, and regulatory compliance
• Minimal operator training required
• Portable, battery-powered design with wireless connectivity
• Seamless integration into modern quality-control workflows and data systems



